Navigating the Patent Dance and Waves of Litigation for Biosimilars
4/8/20243 min read
The Patent Dance and the Wave of Litigation for Biosimilars
In the world of biopharmaceuticals, biosimilars have gained significant attention in recent years. A biosimilar is a biological product that is highly similar to and has no clinically meaningful differences from an FDA-approved reference biological product. The development and approval of biosimilars involve a complex process that includes navigating through patent rights and potential litigation.
The Choices for Biosimilar Applicants
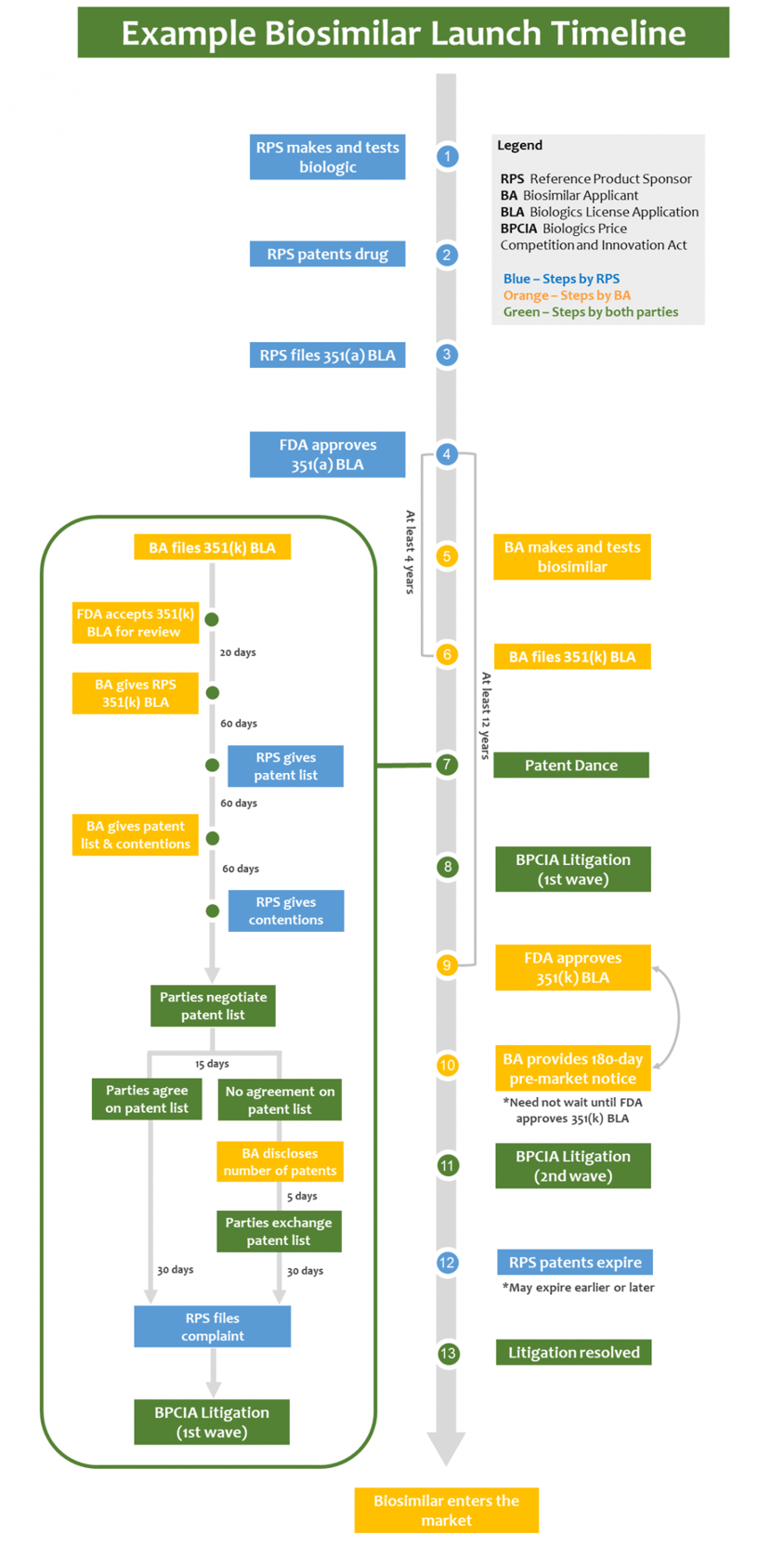
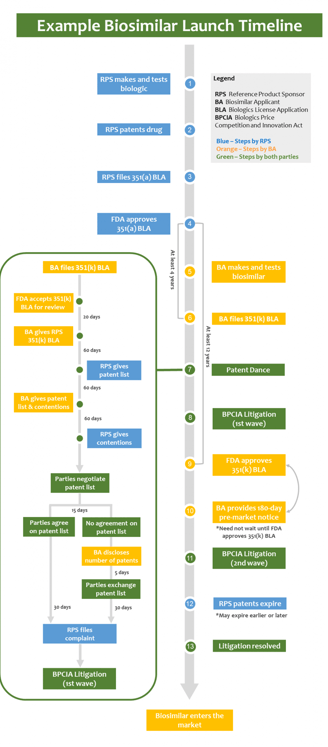
When a biosimilar applicant decides to bring a biosimilar product to the market, they have several choices to make. One of the first decisions is whether to launch the product "at risk" or attempt to clear the patent rights controlled by the reference product sponsor (RPS) before launch. Clearing the patent rights is a crucial step to avoid potential infringement claims and litigation.
The Patent Dance
If a biosimilar applicant chooses to clear the RPS's patent rights, they may engage in a procedure known as the "patent dance." The patent dance is a series of exchanges between the biosimilar applicant and the RPS, intended to resolve any patent disputes before the biosimilar product is launched.
The patent dance begins with the biosimilar applicant providing the RPS with a detailed statement describing how their biosimilar product is similar to the reference product. The RPS then has a specific period of time to provide the biosimilar applicant with a list of patents that they believe could be infringed upon by the biosimilar product.
After receiving the list of patents, the biosimilar applicant has a limited time to respond and provide their own analysis of the patents. This exchange of information allows both parties to evaluate the potential patent disputes and potentially reach an agreement on which patents are valid and infringed upon.
However, the patent dance is not mandatory, and biosimilar applicants have the option to skip this process altogether. By choosing not to engage in the patent dance, the biosimilar applicant may face increased uncertainty and potential litigation from the RPS.
Multiple Waves of Litigation
In addition to the patent dance, biosimilar applicants may also face multiple waves of litigation. These waves of litigation can occur even after the patent dance process has been completed or skipped altogether.
The first wave of litigation typically involves the RPS filing a lawsuit against the biosimilar applicant for patent infringement. This lawsuit initiates a legal battle between the two parties, where the validity and infringement of the patents are examined by the court.
If the biosimilar applicant is successful in defending their product's validity and non-infringement, they may proceed with launching their biosimilar product. However, if the court finds in favor of the RPS, the biosimilar applicant may be subject to an injunction, preventing them from launching their product until the patent expires or is otherwise invalidated.
In some cases, even after the first wave of litigation is resolved, there may be subsequent waves of litigation involving additional patents or challenges to the initial court decision. These subsequent waves can prolong the legal battle and further delay the launch of the biosimilar product.
The Impact on Biosimilar Market Entry
The patent dance and the wave of litigation for biosimilars have significant implications for the entry of biosimilar products into the market. The complexity and uncertainty surrounding patent rights and potential litigation can deter biosimilar applicants from pursuing the development and launch of their products.
Additionally, the costs associated with the patent dance and multiple waves of litigation can be substantial. Biosimilar applicants need to allocate resources for legal representation, patent analysis, and potential damages in case of an unfavorable court decision.
Furthermore, the potential delays caused by the patent dance and litigation can impact patient access to more affordable biosimilar alternatives. These delays may result in higher healthcare costs and limited competition in the market.
Conclusion
The patent dance and the wave of litigation present significant challenges for biosimilar applicants seeking to bring their products to the market. Navigating through patent rights and potential litigation requires careful consideration and strategic decision-making.
While the patent dance offers a potential avenue for resolving patent disputes, it is not mandatory, and biosimilar applicants have the choice to skip this process. However, by choosing to skip the patent dance, they may face increased uncertainty and potential litigation.
The multiple waves of litigation that can follow the patent dance further complicate the process of bringing biosimilar products to market. These waves of litigation can delay market entry and increase costs for biosimilar applicants.
Overall, the patent dance and the wave of litigation highlight the complexities and challenges in the development and approval of biosimilars. As the biosimilar market continues to grow, it is essential for regulators and stakeholders to address these challenges and ensure a fair and efficient pathway for biosimilar entry into the market.